
此文件來自 台灣畜產種原知識庫
https://agrkb.angrin.tlri.gov.tw
The Japanese Quail
Makoto MIZUTANI
Laboratory Animal Research Station,Nippon Institute for Biological Science, Kobuchizawa, Yamanashi, Japan, 408-0041
The Japanese quail belongs to the order Galiformes, genus Coturnix, and species japonica. The scientific designation for Japanese quail is Coturnix japonica, different from the common quail (Coturnix coturnix). The Japanese quail is found in Japan, Korea, Eastern China, Mongolia and Sakhalin as migrating birds.
The plumage color of the wild type is predominantly dark cinnamon brown. However, adult females have pale breast feathers that are speckled with dark colored spots. Adult males have uniform dark rust-red feathers on the breast and cheek (Fig. 1). Sex differences in plumage color appear about 3 weeks of age but not at 0 – weeks of age.
The eggshell color of the wild quail is white, flesh-tint, light brown or speckled blue and/or brown. The size, shape and color pattern on the eggs vary considerably among females. These differences in egg color have been proposed as a means of identifying hens.
The Japanese quail is only one of many animals domesticated by the Japanese. The first record of wild Japanese quail appeared in the eighth century in Japan. Thereafter, several records of wild Japanese quail were found in several eras in Japan. The Japanese quail was semi-domesticated during the sixteenth century as a singing bird. Within the period from 1907 to 1941, the Japanese quail was selected for increased egg production. In 1941, about 2 million Japanese quail were kept. However, the majority of these domesticated quail were lost during the Second World War. After the war, the Japanese quail egg industry was rebuilt from the few remaining domesticated birds, possibly with the addition of domesticated lines from Korea, China, Taiwan and quails captured in the wild. Japanese quail are now farmed mainly for egg production in Japan. In Europe, they are selected for increased body weight for meat production. The body weight of meat quail is 2 – 3 times heavier than that of the egg production type.
Currently, about seven million Japanese quail are kept in Japan. About 4 million birds are kept in Toyohashi City in Aich prefecture. About 70 percent of the eggs are produced in this area. Recently, sex-linked brown plumage color has been used for auto-sexing in the quail industry in Toyohashi.
As mentioned above, the Japanese quail was used first as a singing bird and then as an egg producer. It later became popular as a laboratory animal, because of its small body size, little consumption and rapid maturation. Some standard biological data are shown in Table 1.
In Japan many Japanese quail strains were established and derived from commercial birds. Some of them are also registered in the International Registry of Poultry Stocks (Somes, 1988). These strains are classified into the following five groups with characteristics described as follows:
I. Random bred closed colonies in which as many varieties are maintained as possible.
I-1. WE strain: A random-bred white eggshell strain with wild plumage color was established in 1972 and maintained at the Nippon Institute for Biological Science (NIBS).
I-2. WT strain: A random-bred colored eggshell strain with wild type plumage color maintained at Hiroshima University.
I-3. Commercial strain: A random-bred colored eggshell strain with wild type plumage color maintained at the National Institute of Livestock and Grassland Science.
I-4. Wild strain: A random-bred colored eggshell strain with wild type plumage color derived from wild Japanese quail captured at Mt. Fuji. This strain is maintained at the National Institute of Livestock and Grassland Science.
I-5. Normal strain: A random bred colored eggshell strain with wild type plumage color maintained at Okayama University.
II. Closed colonies in which some specific marker genes (plumage color, eggshell color, blood typing et al.) are fixed.
II-1. PS strain: A random bred autosomal recessive pansy plumage strain maintained at Hiroshima University (Tsudzuki and Wakasugi, 1987). The pansy chicks show light yellow down with three narrow black stripes on the back. The adult plumage is basically composed of three colors, i.e. rust, black and white. Black is predominant on the head. The male has a brown or heavy rust face. The female shows a wheat-straw colored face with black stripes. In the back feathers, color bands are arranged from the base to the tip as follows: a gray base, mixed area of rust and black, large black area, a rust area and white tip. The rachis color is white at the base and becomes dark toward the tip.
II-2. YWE strain: Established in 1974. A random bred white eggshell strain maintained at NIBS. Segregated for autosomal homolethal yellow plumage color gene.
II-3. AMRP strain: Established in 1974 and maintained at NIBS by random breeding in a closed colony. Selected using natural agglutinin for mouse red blood cells. This strain is homozygous for blood group system with following loci by lectins: Sn/Sn (Glycine soja) (Mizutani et al., 1977a, b), Ns/Ns (fruits bodies of Naematoloma sublaitum) (Mizutani et al., 1981). The genes of Es-Da isozyme in red blood cells and autosomal recessive plumage color panda(s) of this strain are homozygous too. The Panda is a spotted plumage color mutant shown in Fig. 2 (Mizutani et al., 1974). The chicks are primarily white, however, wild type down or feathers are sprinkled around the eyes and ears and on the head, back, tail, secondary flight and covert feathers.
II-4. SBPN strain: Established in 1973 by pedigree breeding at NIBS and maintained to date by random breeding in a closed colony. This strain is homozygous for blood group systems with the following loci by lectins: sn/sn (Glycine soja), ns/ns (fruits bodies of Naematoloma sublatium), and for the autosomal recessive plumage color panda gene(s).
II-5. PNN strain: Established in 1972 and maintained at NIBS as a random-bred closed colony with colored eggshells and wild type plumage. The strain is homozygous for blood group systems with Ly2/Ly2 locus by isoimmune serum (Katoh and Wakasugi, 1981) and the following loci by lectins: pn/pn (Peanut) (Mizutani et al., 1977), Sn/Sn, and ns/ns.
II-6. TKP strain: Obtained from the Takeda Co. in 1983 and maintained at NIBS as a random-bred closed colony with colored eggshells and wild type plumage. This strain is homozygous for blood group system with Ly3/Ly3 locus by isoimmune sera, following loci by lectins: Sn/Sn and Ns/Ns, and for Es-Db isozyme of red blood cells.
II-7. GUC strain: Obtained from Gifu University in 1994 and maintained at NIBS. The strain is homozygous for autosomal recessive eggshell color celadon (ce) (Ito et al., 1993). The eggshell color resembles Sung era porcelain. A spectrophotometric analysis revealed that celadon eggshell pigments are protoporphyrin and biliverdin as in the wild type eggshell, but the protoporphyrin content is much lower than that in the wild type. The biliverdin content was 44% of the wild type. A quail with both genes of eggshell color celadon and white homozygously lays a pure white egg (Fig. 3).
III. Closed colonies used as animal models for human hereditary diseases, malformations and abnormalities controlled by one or few genes.
III-1. S strain
Mutant genes: silver plumage, B, and the Amy-2A, are kept in the strain. Silver is controlled by an autosomal incomplete dominant gene. Homozygous quail show white plumage and a retinal defect (ring retina). A central part of the retina lacks pigment (Homma et al., 1969). Grossly, a circular area of hypopigmentation in the posterior retina with thinning in the subjacent sclera is observed in all B/B homozygous quails. As the birds mature, the thinned sclera progresses to scleral ectasia. Posterior scleral ectasia may have developed secondarily to a congenital structural defect in the posterior portion of the sclera associated with general ocular defects. Heterozygous quail show grayish plumage except for several white feathers on the wing tip. Recently, silver homozygous quail were reported to have mutations in the Mitf gene. The Mitf gene in B/B homozygous quail impair osteoclastic bone resorption (Kawaguchi et al., 2001). The same mutant is kept at NIBS as a GUB strain (Fig. 4).
III-2. DFND (dark feather nervous disorder) strain
The mutant is controlled by an autosomal recessive gene. The plumage of chicks and adults is dark brown and the primary and secondary feathers are frayed. The mutant is characterized by cerebellar functional disorders involving fine tremors in the extremities and trunk, gait disturbance and tumbling when the quail is active or excited. The mutant quail shows smaller cerebellum profiles than the normal. In the mutant’s cerebellum, the Purkinje cell soma is small and fusiform in shape, independent of its location. In the normal quail, the Purkinje soma is flask-shaped. Purkinje cells in the mutant are more intensely stained in the Bodian and Nissl preparations. The mean cell number per unit area of Purkinje cells and granular cells is not different from the normal quail. This mutant is similar to the Reeler mouse in histopathology and clinical features. However, this mutant lacks Golgi II neurons in the white matter of the cerebellum as observed in the Reeler mouse (Ueda et al., 1979).
III-3. RWN strain
Established in 1976 and maintained at NIBS. Glycogen storage disease type II (acid maltase deficiency, AMD) appears in all quails in this strain. The AMD is controlled by an autosomal recessive gene. The AMD quail symptoms involve wing dysfunction (Fig. 5). Histopathologically, the cells in the liver, heart and skeletal muscles show cytoplasm with decreased staining by hematoxylin and eosin. PAS-positive material deposition is observed in the liver, heart, skeletal muscles and to a lesser extent in the brain, intestine and gizzard. This material is digested by diastase in all affected tissues and considered glycogen. The superficial pectralis muscle is most severely affected in the skeletal muscle. With 4-MUG as the substrate, the acid maltase activity is decreased in the femoral muscle, superficial pectral muscle, heart muscle and liver of affected quail. However, there is no difference in the neutral maltase activity in those tissues (Murakami et al., 1982).
We attempted to establish an early onset strain by mating quails that showed wing dysfunction at 3 weeks of age. However, we occasionally found some birds with normal behavior at 10 weeks of age in every generation. From original early onset AMD quails (RWNE strain), we isolated late onset AMD quails (RWNL strain) with a normal wing functions in spite of the presence of pathological changes in the pectral and wing muscle. So far, late onset AMD quails have not exhibited the severe wing dysfunction found in early onset AMD quails even after 35 weeks of age. The segregation ratio for the early onset type and late onset type in the RWNE strain is 2:1. The RWNL strain produces only the late onset type AMD quail. The hatchability of the RWNE strain is always lower than that of the RWNL strain. These results indicate that a dominant modifier gene influences the severity of AMD in Japanese quail. Namely, quail that have a dominant modifier gene homozygously die before hatching. The heterozygous quail show early onset type AMD. The recessively homozygous quail show late onset type AMD. Histopathological analysis could not detect the cause of death in quail embryos that had a homozygous dominant modifier gene. These three types of AMD quail resemble the human infantile form, child form and adult form, respectively.
Enzyme replacement therapy for AMD quail using human recombinant GAA showed a decrease in glycogen in the heart and liver (Kikuchi et al., 1998). In 1997, the full-length acid maltase cDNA of Japanese quail was isolated from cDNA library derived from Japanese quail liver (Kunita et al., 1997).
III-4. LWC strain
Myotonic dystrophy quail appear in two third of the quail in this strain. The mutant is controlled by an autosomal dominant homo lethal gene. The disorder is clinically apparent as early as 28 days of age. It is characterized by generalized myotonia, muscle stiffness and muscle weakness. Affected birds are identified by their inability to lift their wings vertically upward and by their inability to right themselves when placed on their dorsum. This symptom is very similar to that in AMD quail (Braga et al., 1995).
Electromyographic studies in mutant quail show high-frequency repetitive discharges comparable to those in myotonic runs. These discharges persist after nerve resection. The distinctive histopathlogic changes in the various muscles examined are ring fibers, sarcoplasmic masses and internal migration in the sarcolemmal nuclei. A slight decrease in the size of type IIB muscle fibers and a slight increase in the size of the type IIA fibers are observed in the M. pectralis thoracicus of affected quails (Fig. 6). The typical muscle lesions and multi-system involvement, manifested by testicular degeneration and atrophy in affected male quail specimens and bilateral lenticular cataracts in 6 of 13 affected quail, suggest the resemblance of this inherited muscular disorder to myotonic dystrophy in humans. The relationship between the development of muscle lesions and age, as well as immunohistochemical and ultrastructural features in this mutant quail are described in reference (Tanaka et al., 1996).
III-5. Quv strain
Neurofilament deficiency appears in all quail in this strain. The mutants show head or body quivering, or both. This trait is characterized by neurofilament deficiency in the axons of the cervical spinal cord and the optic and sciatic nerves, and named “hypotrophic axonopathy”. This characteristics is controlled by an autosomal recessive gene (Mizutani et al., 1992). This is the first mutant suggesting compatibility without neurofilaments (NFs) in a vertebrate. The existence of this mutant showed that NFs are not necessary to the maintenance of life.
The mutant strain (Quv) was successfully established. Electron microscopically and immunohistochemically, neurofilaments are not detected in the axons or neuronal cell bodies in Quv. Axons in Quv are composed mainly of microtubules, which are increased in number in relation to the axonal size (Fig. 7). Gel electrophoresis and Western blot analysis indicate that the low, middle and high molecular mass neurofilament subunits are markedly deficient in the brain, cervical spinal cord and sciatic nerve of Quv. Immunohistochemically, the Quv spinal cord has no immunoreactive products corresponding to low molecular mass NF. However, the middle and high molecular mass NF antisera stained axons (Yamasaki et al., 1992).
We hypothesize that the NF deficiency in Quv results from an alteration in the filament assembly caused by defective expression of low molecular mass NF (NF-L). When the NF-L gene sequences of normal and mutant quail were compared, the NF-L gene in the mutant was found to have a nonsense mutation at the deduced amino acid residue 114. This indicates that the mutant is incapable of producing even a trace amount of NF-L protein in any situation. The morphological features of the myelinated fibers in Quv were also studied in detail (Zaho et al., 1995). Furthermore, the neurotoxic effects of acrylamide (Takahashi et al., 1995), β-β’-iminodipropionitrile (Mitsuishi et al., 1993) and 2,5-Hexanedione (Hirai et al., 1999) were also investigated using the Quv strain.
III-6. ET strain
This strain was found in the B strain maintained at Nagoya University, and is now maintained at Hiroshima University. Ear tufts and irregular shaped ear openings appear in all quail in this strain. The mutant ear opening is oval shaped with a fissure on its ventral margin. Ear tufts project from the ventral end of this fissure or the posterior margin of the ear opening. The ear tufts are composed of a feathered peduncle. The size of the ear tufts and the ear-opening abnormality are variable. In 5-day old ET strain embryos, the incidence of an incomplete closure of the hyomandibular furrow is 91%. This hyomandibular furrow abnormality seems to be the primary defect leading to the ear opening and ear-tuft traits. This characteristics is controlled by an autosomal recessive gene (Tsudzuki and Wakasugi, 1988).
III-7. TT strain
In 1985, two chicks exhibiting throat-tufts (TT) were found in the Japan-Taiwan mixture (JTM) strain maintained at Nagoya University. The TT strain is now maintained at Hiroshima University.
The throat-tuft phenotype in Japanese quail is characterized by a throat tuft consisting of an epidermal peduncle and covering feathers. Sometimes it is accompanied by abnormal ear openings showing a wide cleft at the ventral margin and, in some instances, an extra bony projection on the peduncle or in its vicinity (Fig. 8). The incidence of throat tufts and ear opening abnormalities in 15-day embryos in the TT strain are 50% and 17%, respectively. The 5-day TT embryos exhibited peduncle formation and hyomandibular furrow defects that are thought to cause throat tuft and ear opening abnormalities, respectively. Mating experiments suggest that the throat tuft characteristic is controlled by an autosomal recessive gene that is allelic and dominant to the ear tuft gene (Tsudzuki and Wakasugi, 1989, 1990).
III-8. HMM strain
Hereditary multiple malformation (HMM), a new mutation in Japanese quail (Fig. 9), was discovered among the progeny from a pair from the WT strain kept at Osaka Prefecture University, now maintained at Hiroshima University.
This characteristics is controlled by an autosomal recessive gene. The majority of the homozygotes die on the sixth day of incubation and the remaining die at various stages until 15 days of incubation. The homozygotes surviving to the late embryonic stages have greatly shortened lower and upper beaks that are set apart and show an early embryo-like body shape, with feather buds but no plumules. Furthermore, they show syndactylous polydactyly in both the fore and hind limbs. In the abdomen of the homozygotes, a portion of the ventriculus, liver and small intestine protrudes out of the umbilicus region. In the skeleton of the late HMM embryos, ossification is generally delayed and morphogenetic abnormalities are observed all over the body. This mutant has become a useful animal model in the morphogenesis research field (Tsudzuki et al., 1998).
III-9. SLB strain
Abnormal embryos with a short lower beak (SLB) were discovered among the progeny from a pair from the DDL strain maintained at Osaka Prefecture University, now maintained at Hiroshima University.
The SLB individuals are characterized by a shortened lower beak, opened mouth and small body size. These mutants die in the late embryonic stages. Skeletal analyses revealed that the Meckel’s cartilage of the mandible is abnormally bent downward in its proximal portion. The mandibular bones are formed around the abnormal Meckel’s cartilage, which seems to be responsible for the shortened lower beak and the opened mouth. The appendicular bones in the mutant embryos are also shortened. Skeletal abnormalities are also observed in the hyoid apparatus, ribs and cervical vertebrae. Genetic analyses revealed that the SLB mutation is controlled by two linked autosomal recessive genes with a recombination rate of 33%. This mutant might be a useful animal model in the study of morphogenesis of the mandible, hyoid apparatus, cervical vertebrae, ribs and appendicular bones (Nakane and Tsudzuki, 1998a; Nakane, 1998).
III-10. ZN strain
Abnormal embryos (Zazen) showing crossed legs and opened eyes were discovered among the progeny from a pair from the SBK strain maintained at Osaka Prefecture University, now maintained at Hiroshima University. The name of the mutant, Zazen, is based on a leg condition similar to the religious mediation figure (=za-zen) in the Zen sect. Zazen birds are characterized by crossed legs, opened eyes and abnormal beaks. In this skeletal system, all mutant embryos have a full-length fibula in both legs from the knee to the hock joint. The femur is articulated with the fibula, but not the tibia. Other skeletal abnormalities are observed in the premaxilla, parietal, and ribs. All Zazen mutants die in the late embryonic stages. Genetic analyses reveal that the mutation is controlled by two genes, autosomal dominant gene and sex-linked recessive gene. The latter is epistatic to the former. This mutant might be a useful animal model in the study of developmental leg skeleton aspects in relation to the genes controlling morphogenesis (Nakane and Tsudzuki, 1998b; Nakane, 1998).
IV. Closed colonies that were established by successive selections of specific characteristics controlled by many genes.
IV-1. LAP strain
Hyperlipidemia atherosclerosis-prone (LAP) strain susceptible to experimental atherosclerosis was established by repeated breeding of highly susceptible quails induced by a diet containing 1% cholesterol. The LAP strain was derived from the HAP strain established by Ohtsuka Pharmaceutical Co. Ltd. The following findings are described.
(1) The retarded rate of cholesterol biosynthesis or catabolism is not responsible for hypercholesterolemia (Nagata et al., 1996).
(2) Cholesterol feeding had no effect on the level of cholesterol, chemical composition or fatty-acid composition of the high-density lipoprotein fractions in LAP and normal strain. Although the lipoprotein and apoprotein profiles in LAP quail show resemblance to those of normal quail, expression of the 470 kDa protein in the lipoprotein appears to be pronounced in the LAP quail (Nagata et al., 1997).
(3) The tissue distribution for apolipoprotein A-I (apo A-I) is similar in both LAP and normal strains, but LAP quail expresses more apo A-I-mRNA than normal quail in all tissues examined (Iwasaki et al., 1999).
(4) The DNA sequence of LAP apo A-I cDNA is similar to that in the normal quail (Iwasaki et al., 1999).
(5) The structure and expression of the major low molecular weight apoprotein has no relevance to higher LAP susceptibility to experimental atherosclerosis (Iwasaki et al., 2000).
IV-2. H2 and L2 strains
The High responder strain (H2) and low responder strain (L2) were established by selective breeding experiments through 50 generations for high and low responders to inactivated Newcastle disease virus (NDV) antigen at National Institute for Environmental Science (NIES) (Takahashi et al., 1984). Hemagglutination inhibition titers of the H2 strain and L2 strain in F18 are 8.54log2 and 1.85log2, respectively. The mortality of the H2 strain after NDV challenge was lower than that for the L2 strain. The H2 strain is a high responder when they are immunized with influenza virus, sheep red blood cells and S. pullorum. There is no correlation between antibody production and reproduction. The mitogenic response of lymphocytes to PHA is higher in the H2 strain than the L2 strain. The rate of spontaneous rosette cells in blood lymphocytes with either rabbit or fowl erythrocytes in the H2 strain were higher than that in the L2 strain (Inooka et al., 1984).
IV-3. LL, SS, RR and SL strains
Three strains for either large (LL) or small (SS) body size at 6 weeks of age and random-bred control strain (RR) were developed by successive selection of 80 generations at Saga University. At 12 weeks of age, the body weights of LL, SS and RR strain are 212.0 g, 66.5 g and 104.8 g, respectively. Researches using above strains are as follows:
(1) Comparative studies on body weight, tibia length and abdominal weight (Ardiningsasi et al., 1992).
(2) Investigation of the feed conversion to body weight gain and egg production (Okamoto et al., 1989).
(3) Genetic studies on muscle protein turnover rate, acid protease activity and calcium-activated neutral protease activity (Maeda et al., 1986; Maeda et al., 1989; Maeda et al., 1991).
(4) Research on the heterosis effects existing in the productive traits (weekly body weight, egg laying traits, fertility, hatchability, rate of raising and viability) (Piao et al., 2002).
The SL strain was derived from the SS strain by selecting large body size. The SS and SL strains would be useful for research on body size miniaturization from molecular biologic and metabolic aspects to provide an insight into the evolutionary mechanism (Suda et al., 2002).
V. Closed colonies that are used in auto-sexing of newly hatched chicks.
V-1. AWE strain
This strain was established in 1975 at NIBS, and is a random-bred white eggshell strain with a homozygous sex-linked albino gene. As the AWE strain was previously crossed with the WE strain; the genetic background of these two strains is similar. These strains are used to investigate the screening and testing of endocrine disruptors on the gonads of Japanese quail. It is possible to check the sex of chicks and embryos using their plumage color when a male AWE quail is mated with female WE quail (Fig. 10).
The strain was established at NIBS and is a random-bred white eggshell strain segregated for sex-linked brown, sex-linked cinnamon and autosomal yellow plumage color genes.
V-3. SBC strain
The strain was established at Hiroshima University. The sex-linked brown and the sex-linked cinnamon genes are segregated.
Acknowledgements
The author thanks Dr. K. Yamanouchi for reading the manuscript.
References
Ardiningsasi, S. M., Maeda, Y., Okamoto, S., Okamoto, S. and Hashiguchi, T. 1992. Comparative studies of body weight, tibia length and abdominal fat weight among lines selected for body size in Japanese quail, Coturnix coturnix japonica. Jpn. Poult. Sci. 29: 310-315.
Braga, III, I. S., Tanaka, S., Kimura, T., Itakura, C. and Mizutani, M. 1995. Inherited muscular disorder in mutant Japanese quail (Coturnix coturnix japonica): relationship between the development of muscle lesions and age. J. Com. Path. 113: 131-143.
Hirai, T., Mizutani, M., Kimura, T., Ochiai, K., Uemura, T. and Itakura, C. 1999. Neurotoxic effects of 2,5-hexanedione on normal and neurofilament-deficient quail. Toxicologic Patholgy 27: 348-353.
Homma, K., Jinno, M. and Kito, J. 1969. Studies on silver-feathered Japanese quail. Jpn. J. Zootech. Sci. 40: 129-130.
Inooka, S., Takahashi, S., Takahashi, H. and Mizuma, Y. 1984. Immunological traits in generations 7 to 12 of two lines of Japanese quail selected for high or low antibody response to Newcastle disease virus. Poultry Sci. 63: 1298-1302.
Ito, S., Tsudzuki, M., Komori, M. and Mizutani, M. 1993. Celadon: An eggshell color mutation in Japanese quail. J. Hered. 84: 145-147.
Iwasaki, H., Oku, H., Toda, T., Nasu, T., Miyagi, T. and Chinen, I. 1999. Apolipoprotein A–I of hyperlipidemia atherosclerosis-prone (LAP) quail: cDNA sequence and tissue expression. Biosci. Biotechnol. Biochem. 63: 29-34.
Iwasaki, H., Oku, H., Toda, T., Nasu, T., Miyagi, T. and Chinen, I. 2000. The major low molecular weight apolipoprotein from normal and hyperlipidemia atherosclerosis-prone (LAP) Japanese quail. Biochimica et Biophysica Acta 1483: 316-324.
Katoh, H. and Wakasugi, N. 1981. Studies on the blood groups in the Japanese quail. Detection of three antigens and their inheritance. Immunogenetics 13: 109-114.
Kawaguchi, N., Ono, T., Mochii, M. and Noda, M. 2001. Spontaneous mutation in Mitf gene causes osteopetrosis in silver homozygote quail. Dev. Dyn. 220: 133-140.
Kikuchi, T., Yang, H. W., Pennybacker, M., Ichihara, N., Mizutani, M., Hove, J. L. K. and Chen, Y. T. 1998. Clinical and metabolic correction of Pompe disease by enzyme therapy in acid maltase-deficient quail. J. Clin. Invest. 101: 827-833.
Kunita, R., Nakabayashi, O., Wu, J. Y., Hagiwara, Y., Mizutani, M., Pennybacker, M., Chen, Y. T. and Kikuchi, T. 1997. Molecular cloning of acid α-glucosidase cDNA of Japanese quail (Coturnix coturnix japonica) and the lack of its mRNA in acid maltase deficient quails. Biochimica et Biophysica Acta 1362: 269-278.
Maeda, Y., Fujii, M., Okamoto, S. and Hashiguchi, T. 1989. Variation among lines selected for body size in the fractional rate of degradation of protein and acid protease activity in the muscle of quail (Coturnix coturnix japonica). Biochemical Genetics 27: 603-611.
Maeda, Y., Hayashi, K., Hashiguchi, T. and Okamoto, S. 1986. Genetic studies on the muscle protein turnover rate of Coturnix quail. Biochemical Genetics 24: 207-216.
Maeda, Y., Kawabe, K., Okamoto, S., Okamoto, S. and Hashiguchi, T. 1991. Genetical studies on muscle protein turnover rate and calcium activated neutral protease activity in the skeletal muscle of the Japanese quail (Coturnix coturnix japonica). Anim. Sci. Technol. (Jpn), 62: 813-821.
Mitsuishi, K., Takahashi, A., Mizutani, M., Ochiai, K. and Itakura, C. 1993. β,β’ iminodipropionitrile toxicity in normal and congenitally neurofilament deficient Japanese quails. Acta Neuropathol. 86: 578-581.
Mizutani, M., Chino, K., Umezawa, H. and Kuramasu, S. 1974. Genetic analysis of a new plumage panda in Japanese quail (in Japanese with English summary). Exp. Anim. 23: 59-61.
Mizutani, M., Nunoya, T., Yamasaki, H. and Itakura C. 1992. The hypotrophic axonopathy mutant in Japanese quail. J. Hered. 83: 234-235.
Mizutani, M., Saito, T., Umezawa, H. and Kuramasu, S. 1981. A new agglutinogen detected by Naematoloma sublatium PHA in Japanese quail. ABRI(Animal Blood Group Research Information) 9: 43-45.
Mizutani, M., Umezawa, H. and Kuramasu. S. 1977a. Studies of the red blood cell agglutinogen in Japanese quail detected by soybean PHA. Jpn. J. Zootech. Sci. 48: 227-234.
Mizutani, M., Umezawa, H. and Kuramasu, S. 1977b. Studies on the hemagglutinogen in Japanese quail detected by peanut PHA. Jpn. J. Zootech. Sci. 48: 463-467.
Murakami, H., Takagi, A., Nonaka, I., Ishiura, S., Sugita, H. and Mizutani, M. 1982. Type 2 glycogen storage disease in Japanese quail. In Muscular Dystrophy (Edited by S. Ebashi), Tokyo Univ. pp. 37-48.
Nagata, J.,Maeda, G., Oku, H., Toda, T. and Chinen, I. 1997. Lipoprotein and apoprotein profiles of hyperlipidemic atherosclerosis-prone Japanese quail. J. Nutr. Sci. Vitaminol. 43: 47-57.
Nagata, J., Oku, H., Toda, T. and Chinen, I. 1996. Effect of dietary choresterol on the activities of key enzymes of choresterol metabolism in hyperlipidemia and atherosclerosis-prone Japanese quail. J. Nutr. Sci. Vitaminol. 42: 287-300.
Nakane, Y. 1998. Exploitation of new animal models in Japanese quail (Coturnix coturnix). Establishment of embryonic skeletogenesis stages and analyses for newly discovered morphogentic mutations. Doctorial thesis, Osaka Prefecture University.
Nakane, Y. and Tsudzuki, M. 1998a. Morphological and genetic studies for new mutant of Japanese quail. In: Proceedings of the 6th Asian Pacific Poultry Congress (Nagoya, Japan), pp. 242-243.
Nakane, Y. and Tsudzuki, M. 1998b. A new morphological lethal mutation showing eye, beak, and leg abnormalities in Japanese quail. In: Proceedings of the 8th World Conference on Animal Production (Soul, Korea), Vol. II, pp. 148-149.
Okamoto, S., Kobayashi, S. and Matsuo, T. 1989. Feed conversion to body weight gain and egg production in large and small Japanese quail lines selected for 6-week body weight. Jpn. Poult. Sci. 26: 227-234.
Piao, J., Okamoto, S., Kobayashi, S., Wada, Y. and Maeda, Y. 2002. Study of heterosis effects on production traits of Japanese quails. 2) Heterosis effects on the crosses between small line and random bred population. Jpn Poult. Sci. 39: 139-146.
Somes, R. G. Jr. 1988. International Registry of Poultry Stocks, Storrs Agricultural Experiment Station, The University of Connecticut, Storrs, Connecticut.
Suda, Y., Imakawa, K. and Okamoto, S. 2002. Long term selection for small body weight in Japanese quail. I: direct selection response from 60 to 65th generation. Jpn. Poult. Sci. 39: 274-284.
Takahashi, S., Inooka, S. and Mizuma, Y. 1984. Selective breeding for high and low antibody responses to inactivated Newcastle disease virus in Japanese quails. Poultry Sci. 63: 595-599.
Takahashi, A., Mizutani, M. and Itakura, C. 1995. Acrylamide-induced peripheral neuropathy in normal and neurofilament-deficient Japanese quails. Acta Neuropathol. 89: 17-22.
Tanaka, S., Braga, III, I. S., Kimura, T., Ochiai, K., Itakura, C. and Mizutani, M. 1996. Inherited muscular disorder in mutant Japanese quail (Coturnix coturnix japonica): an ultrastructural study. J. Com. Path. 114: 325-337.
Tsudzuki, M., Nakane, Y. and Wada, A. 1998. Hereditary multiple malformation in Japanese quail: a possible powerful animal model for morphogenetic studies. J. Hered. 89: 24-31.
Tsudzuki, M. and Wakasugi, N. 1987. “Pansy”: a plumage color mutant in Japanese quail. Jpn. Poult. Sci. 24: 327-335
Tsudzuki, M. and Wakasugi, N. 1988. A genetic defect in hyomandibular furrow closure in the Japanese quail: The causes for ear-opening abnormality and formation of ear tuft. J. Hered. 79: 160-164.
Tsudzuki, M. and Wakasugi, N. 1989. Throat tuft: A new discovered morphological mutation in Japanese quail. J. Hered. 80: 433-441.
Tsudzuki, M. and Wakasugi, N. 1990. A genetic analysis on the throat-tuft character of Japanese quail (Coturnix coturnix japonica) based on the head-skeleton abnormality. Jpn. Poult. Sci. 27: 393-397.
Ueda, S., Ito, H., Masai, H. and Kawahara, T. 1979. Abnormal organization of the cerebellar cortex in the mutant Japanese quail, Coturnix coturnix japonica. Brain Research 177: 183-188.
Yamasaki, H., Bennett, G. S., Itakura, C. and Mizutani, M. 1992. Defective expression of neurofilament protein subunits in hereditary hypotrophic axonopathy of quail. Lab. Invest. 66: 734-743.
Zaho, J. X., Ohnishi, A., Itakura, C., Mizutani, M., Yamamoto, T., Hojo, T. and Murai, Y. 1995. Smaller axon and unaltered numbers of microtubules per axon in relation to number of myelin lamellae of myelinated fibers in the mutant quail deficient in neurofilaments. Acta Neuropathol. 89: 305-312.
Fig. 1. Japanese quail (Left: female, Right: male)

Fig. 2. Japanese quail with panda plumage

Fig. 3. Egg shell color (we: white, ce: celadon, pw: pure white)

Fig. 4. GUB strain (Silver plumage)

Fig. 5. AMD quail
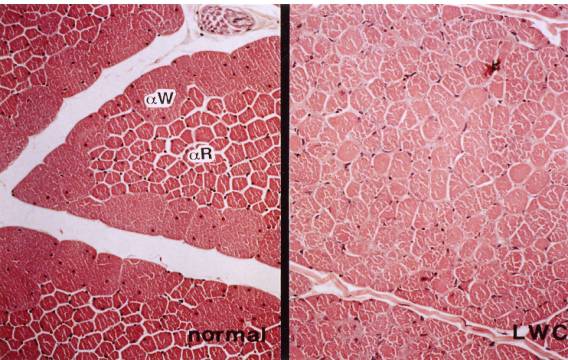
Fig. 6. M. pectoralis thoracicus of normal (left) and LWC (right) quail
Left: Type αW (IIB) and αR (IIA) fibers are well differentiated
Right: Note a slight decrease in the size of the type αW fibers with a relative increase in the size of type αR fibers compared to the normal quail

Fig. 7. Electron microscopy of nerve fiber of normal (left) and Quv (right) quail
Left: axonal cytoskeleton of normal quail comprise a number of NFs with less microtubules
Right: NF central cores were not observed but microtubules were increased in number

Fig. 8. Dotted white quail with a large throat tuft on the left side only (Photo by M. Tsudzuki)
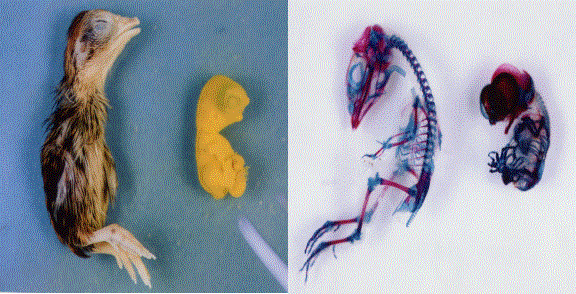
Fig. 9. Comparison of an external appearance and skeleton of 13-days embryo of normal (left) and HMM mutant quail (right) (Photo by M. Tsudzuki)

Fig. 10. Left: Male of AWE strain, Right: Female of WE strain
此文件的網址 :
https://agrkb.angrin.tlri.gov.tw/index.php?page=3271
